Jack Cochran explains how to optimise for speed or match your original compound retention times via the use of a novel translator
When discussing the conversion of GC methods from helium to hydrogen carrier gas, generally the focus is on speed as hydrogen has a higher optimal flow rate than helium and can be used to achieve faster run times without sacrificing separation efficiency.
While speedier analysis times offer the attraction of improved productivity, there are times when matching the original compound retention times is more important, for example, to make calibration updates or new method validation easier.
Regardless of whether the goal is faster analyses or maintaining the original compound retention times, proper method translation is critical for success. The new EZGC method translator/flow calculator from Restek is an easy-to-use tool that ensures proper conversion from helium to hydrogen for either speed optimised or matched retention time scenarios.
Helium, like hydrogen, is a gas at normal temperatures, light enough for party balloons and giving you a squeaky high voice if you inhale it. It was also made in the Big Bang, with more coming from that nuclear fusion reaction in stars. The Basics of Limitless Power: Albert Einstein's famous E=MC2 equation reflects the enormous energy that can be released by fusing atoms. Hydrogen atoms fusing together to create helium powers the.
Increase sample throughput with faster separations
Obtaining faster GC run times so more samples can be analysed in a day is often the driving force behind converting from helium carrier gas to hydrogen. With proper method translation, this can be an easy way to improve productivity and reduce dependence on expensive and increasingly scarce helium.
- The abundance of hydrogen and helium helps scientists understand the universal expansion rate. The scientists believe that if the universe had expanded any faster there would have been more stable neutrons and thus more helium; if it had expanded slower however the temperature would have been much hotter resulting in more unstable neutrons.
- Helium is quite a lot lighter than air: it's about an eighth of the density of air. Hydrogen is about a sixteenth the density of air, so it'll float in air and will in fact float upwards. You'd have thought that hydrogen would be a better gas as it would give slightly more lift than helium because it's lighter.
- Aug 25, 2017 Helium and hydrogen are chemical elements that are mostly found in the atmosphere as gaseous substances due to their very low melting and boiling temperatures. The main difference between Helium and hydrogen is that helium atom exists as a monoatomic gas in the atmosphere whereas hydrogen exists as a diatomic gas in the atmosphere.
The conversion requires a faster GC oven program rate for hydrogen versus helium to maintain the same chromatographic elution pattern for the compounds of interest. For example, when translating a GC-MS pesticides analysis from helium to hydrogen, the conditions for the original method using helium were simply entered into the EZGC method translator and the software returned a translated method. This translated method uses a faster flow rate and oven ramp rate.
As shown in Fig. 1 on the previous page, the translated method yielded a very comparable chromatographic separation with no elution order changes in nearly half the time.
Maintain original retention times for easier calibration updates
In the second scenario, where the goal is to maintain not just the same peak elution order, but also the same retention times as closely as possible, the method conversion is based on using approximately the same linear velocity for both gases, which is best done by matching the holdup time of the new hydrogen carrier method with the helium holdup time from the original method.
Helium Hydrogen Mix
Here, the EZGC method translator is used in custom mode and the holdup time (and/or linear velocity) for hydrogen is set to match that of helium (see Fig. 2).
This means the GC column is operating below the optimum flow rate for hydrogen carrier gas, but an advantage is gained in being able to use exactly the same GC oven program from the original helium method.
Fig. 3 demonstrates that this approach gives essentially the same retention times as were obtained when using helium, with no noticeable loss in separation even though hydrogen is used at a sub-optimum flow.
This technique of matching the linear velocities and holdup times for helium and hydrogen when switching carrier gases can be used to some advantage with GC-MS, where hydrogen is not easily pumped and a higher (optimum) flow would lead to a more drastic detectability loss.
In addition, confirmation of method performance is simpler as the oven program and retention time windows do not change.
This approach should allow easier entry for labs making the switch from helium to hydrogen carrier gas for GC.
Summarising the merits of this approach
In summary, the EZGC method translator has been built specifically for GC method development. It has a number of practical uses, including increasing speed of analysis through decreasing column length and/or decreasing inner diameter and/or switching to a faster carrier gas.
The method translator is also suitable for updating the oven temperature program through translation after column trimming for maintenance so peak elution orders do not change.
Another usage is for improving original methods in separation and/or speed of analysis by solving for efficiency or speed in translation.
In addition, the EZGC method translator can also be used for translating methods from GC-FID (or other atmospheric outlet detector) to GC-MS (vacuum outlet) or vice versa.
To conclude, if you are looking to develop a new GC method or to reliably optimise an existingapplication, Restek’s latest EZGC method development tool can save you hours of calculations, guesswork, and trial-and-error. The free, web-based application is easily accessible and Windows users can download it for offline use.
For more information at www.scientistlive.com/eurolab
Jack Cochran is with Restek.
Next:Hydrogen Molecule IonA helium atom consists of a nucleus of charge surroundedby two electrons. Let us attempt to calculate its ground-state energy.
Let the nucleus lie at the origin of our coordinatesystem, and let the position vectors of the two electrons be and , respectively. The Hamiltonian of the system thustakes the form
| (1181) |
where
In other words, the Hamiltonian just becomes the sum of separate Hamiltonians for each electron. In this case, we would expect thewavefunction to be separable: i.e.,
| (1183) |
Hence, Schrödinger's equation
Helium Hydrogen Fusion Atom
reduces to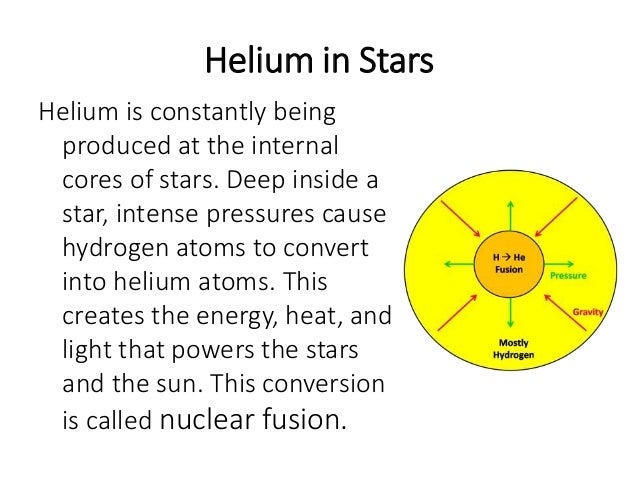
| (1185) |
where
Of course, Eq. (1185) is the Schrödinger equation of a hydrogen atom whosenuclear charge is , instead of . It follows, from Sect. 9.4 (making the substitution ), that if both electrons are in their lowest energystates then
| (1187) |
| (1188) |
where
Here, is the Bohr radius [see Eq. (679)]. Note that is properly normalized. Furthermore,
| (1190) |
where is the hydrogen ground-stateenergy [see Eq. (678)]. Thus, our crude estimatefor the ground-state energy of helium becomes
Unfortunately, this estimate is significantly different from the experimentallydetermined value, which is . This factdemonstrates that the neglected electron-electron repulsion term makes alarge contribution to the helium ground-state energy.Fortunately, however, we can use the variational principle to estimate this contribution.
Let us employ the separable wavefunction discussed above as our trialsolution. Thus,
| (1192) |
The expectation value of the Hamiltonian (1180) thus becomes
where
| (1194) |
The variation principle only guarantees that (1193) yields anupper bound on the ground-state energy. In reality, we hopethat it will give a reasonably accurate estimate of this energy.
It follows from Eqs. (678), (1192) and (1194) that
| (1196) |
where is the angle subtended between vectors and .If we perform the integral in space before that in space then
where
| (1198) |

Our first task is to evaluate the function . Let be a set of spherical polar coordinates in space whose axis of symmetry runs in the direction of . It followsthat . Hence,
| (1200) |
Making the substitution , we can see that
Now,
| (1202) |
giving
But,
| (1204) |
| (1205) |
yielding
Since the function only depends on the magnitude of ,the integral (1197) reduces to
| (1207) |
which yields
Hence, from (1193), our estimate for the ground-stateenergy of helium is
Helium Hydrogen Atoms
| (1209) |
This is remarkably close to the correct result.
We can actually refine our estimate further. The trial wavefunction (1192) essentially treats the two electrons as non-interacting particles. Inreality, we would expect one electron to partially shield the nuclearcharge from the other, and vice versa. Hence, a bettertrial wavefunction might be
We can rewrite the expression (1180) for the Hamiltonianof the helium atom in the form
| (1211) |
where
is the Hamiltonian of a hydrogen atom with nuclear charge ,

| (1213) |
is the electron-electron repulsion term, and
It follows that
| (1215) |
where is the ground-state energy of a hydrogenatom with nuclear charge , is the value of the electron-electron repulsion term whenrecalculated with the wavefunction (1210) [actually, all weneed to do is to make the substitution ], and
Here, is the expectation value of calculatedfor a hydrogen atom with nuclear charge . It follows fromEq. (695) [with , and making the substitution ] that
| (1217) |
Hence,
since . Collecting the various terms, our new expression for the expectationvalue of the Hamiltonian becomes
| (1219) |
The value of which minimizes this expression is the root of
It follows that
| (1221) |
The fact that confirms our earlier conjecture that the electrons partiallyshield the nuclear charge from one another. Our new estimatefor the ground-state energy of helium is
This is clearly an improvement on our previous estimate (1209) [recall that thecorrect result is eV].
Obviously, we could get even closer to the correct value of thehelium ground-state energy by using amore complicated trial wavefunction with more adjustable parameters.
Note, finally, that since the two electrons in a helium atom are indistinguishable fermions, the overall wavefunction must be anti-symmetric with respect to exchange of particles (see Sect. 6).Now, the overall wavefunction is the product of the spatial wavefunctionand the spinor representing the spin-state. Our spatial wavefunction (1210) is obviously symmetric with respect to exchange ofparticles. This means that the spinor must be anti-symmetric. It is clear, from Sect. 11.4, that if the spin-state ofan system consisting of two spin one-half particles (i.e., two electrons)is anti-symmetric with respect to interchange of particles then the system isin the so-called singlet state with overall spin zero. Hence,the ground-state of helium has overall electron spin zero.
Next:Hydrogen Molecule Ion Up:Variational Methods Previous:Variational PrincipleRichard Fitzpatrick2010-07-20
