| Type | Subsidiary |
|---|---|
| Founded | 1999 |
| Founder | Trevor Bentley Mauro Casalese Olaf Miller Rob Davies |
| Headquarters | 123 West 7th Avenue Vancouver, British Columbia V5Y 1L8 |
| 3 (2020) | |
Key people | Jennifer McCarron (CEO) Matthew Berkowitz(CCO) |
| >600 (2019)[1] | |
| Parent | Thunderbird Entertainment |
| Website | http://atomiccartoons.com |
Atomic's first fully original creation, the show's titular heroine served as the studio's mascot for a number of years. In 2010, Miller left to launch his own studio. The following year, Jennifer McCarron was appointed head of production. On July 8, 2015, Atomic Cartoons was acquired by Canadian production company Thunderbird Films. How to say atomic number 13 in English? Pronunciation of atomic number 13 with 1 audio pronunciation, 5 synonyms, 1 meaning, 5 translations and more for atomic number 13.
/digital-man-cradling-atom-538110110-577315d55f9b5858753b0310.jpg)
Atomic Cartoons is a Canadian animation studio founded in 1999 by Trevor Bentley, Mauro Casalese, Olaf Miller, Adam Ronald, and Rob Davies.[2][3] Based out of Vancouver, British Columbia, it produces service animation for a wide variety of clients, as well as creating its own properties. Since 2015, the company has been owned by Thunderbird Entertainment.[4]
History[edit]
The studio was founded in March 1999 by Trevor Bentley, Mauro Casalese, Olaf Miller and Rob Davies.[5] Sent back to Vancouver after losing his job at Warner Bros. Animation following the cancellation of Pinky, Elmyra & the Brain, Davies received a phone call from Sunwoo Entertainment's Jae Moh to help produce Milo's Bug Quest [ko]. Together with former Studio B Productions colleagues Miller and Bentley, as well as animator/character designer Casalese, the four launched Atomic Cartoons to assist in creating the series.[2]
Between 2004 and 2008, the company produced Atomic Betty for Teletoon in association with Breakthrough Entertainment and Tele Images Kids. Atomic's first fully original creation, the show's titular heroine served as the studio's mascot for a number of years.
In 2010, Miller left to launch his own studio. The following year, Jennifer McCarron was appointed head of production.[6] On July 8, 2015, Atomic Cartoons was acquired by Canadian production company Thunderbird Films.[4] The three founders remain on board. McCarron was named president and chief executive officer in 2016.[7]
In December 2018, the company opened a second animation studio in Ottawa, Ontario.[8] Its first project is the Netflix-original The Last Kids on Earth. By June 2020, the Ottawa location is expected to have 130-150 employees.[9]
In February 2020, Atomic Cartoons opened its third studio in Los Angeles, California.[10]
Productions[edit]
| Title | Years | Network | Co-Productions | Notes |
|---|---|---|---|---|
| Spider-Man Unlimited | 1999-2001 | Fox Kids | Marvel Studios Saban Entertainment Koko Enterprise Co., Ltd. Dong Yang Animation | [11] |
| Courage The Cowardly Dog | 1999-2002 | Cartoon Network | Stretch Films | Additional storyboards only |
| Milo's Bug Quest | 1999-2000 | KBS 2TV | Sunwoo Entertainment | Character, prop and background designs, and storyboards[12] |
| Timber Wolf | 2001 | Warnerbros.com | [13] | |
| Max & Ruby | 2002-2019 | Treehouse TV | Nelvana | Based on the book series by Rosemary Wells. Seasons 6-7 only, previously produced by Silver Lining Productions for the first five seasons, 9 Story Entertainment for seasons 3-5 and Chorion for seasons 4-5. |
| Atomic Betty | 2004-2008 | Teletoon M6 (seasons 1–2) Télétoon (season 3) | Breakthrough Entertainment Tele Images Kids Phil Roman Entertainment Marathon Media | [14] |
| Johnny Test | 2005-2014 | Kids' WB (seasons 1-3) Cartoon Network (seasons 4-6) Teletoon | Cookie Jar Entertainment Warner Bros. Animation (seasons 1-2) DHX Media (season 6) | Storyboards for the first season, animation service for seasons 4-6 |
| Captain Flamingo | 2006-2008 | YTV GMA Network | Breakthrough Films and Television Heroic Film Company Philippine Animation Studio Inc. | [15] |
| Click and Clack's As the Wrench Turns | 2008 | PBS | [16] | |
| Babar and the Adventures of Badou | 2010-2015 | YTV TF1 Disney Junior | Nelvana TeamTO LuxAnimation (seasons 1-2) The Clifford Ross Company | [17] Based on the original Babar books by Jean and Laurent de Brunhoff. Computer-animated sequel and spin-off to the original Babar television series. |
| Transformers: Rescue Bots | 2012-2016 | Discovery Family | Darby Pop Productions (season 1) Hasbro Studios | Season 1 only, overtook by Vision Animation and Moody Street Productions for the second season and DHX Media Vancouver for the third and fourth seasons. |
| Rocket Monkeys | 2013-2016 | Teletoon | Breakthrough Entertainment Hornet Films | [18] |
| Ella the Elephant | 2013-2014 | TVOKids | DHX Cookie Jar Inc. FremantleMedia Kids and Family Entertainment | [19] |
- Space Racers (2014–ongoing, uncredited)
- Chub City (2014, scrapped project)
- Little Charmers (2015–2017, in association with Nelvana)
- Pirate Express (2015)
- Five Alarm Funk 'Robot' (2015, music video)
- Nico Can Dance! (2015–ongoing)
- Screechers Wild (2016, Chinese co-production with Alpha Group Co., Ltd.)
- Counterfeit Cat (2016–2017)
- Beat Bugs (2016–ongoing)
- Winston Steinburger and Sir Dudley Ding Dong (2016–2017)
- Marvel Super Hero Adventures (2017–ongoing)
- Minecraft Mini Series (2017–2018)
- Legend of the Three Caballeros (2018, in association with Mercury Filmworks and 6 Point Harness)
- Cupcake & Dino: General Services (2018–2019)
- Super Dinosaur (2018–2019)[20]
- Hilda (2018–ongoing, in association with Mercury Filmworks)
- Lego Jurassic World: The Secret Exhibit (2018)
- 101 Dalmatian Street (2019–2020)
- Lego Spiderman: Vexed by Venom (2019)
- Lego Jurassic World: Legend of Isla Nublar (2019)
- Molly of Denali (2019–ongoing)
- Curious George: Royal Monkey (2019)
- The Last Kids on Earth (2019–ongoing)
- Hello Ninja (2019–ongoing)
- Lego Jurassic World: Double Trouble (2020)
- Lego Star Wars Holiday Special (2020)[21]
- Mighty Express (2020)
- Curious George: Go West, Go Wild! (2020, in association with Universal Animation Studios)
- Lego Avengers: Climate Conundrum (2020)
- Trolls: TrollsTopia (2020 -ongoing)
- Mermicorno (TBA)[22]
- Nate Create (TBA)[23]
- Princesses Wear Pants (TBA)[24]
- Captain Cornelius' Cartoon Lagoon (TBA, television series based on the 2012 short)[25]
- Mariachi Zombie (TBA)[26]

Atomic Number 13 Group Number
References[edit]
- ^Ross, Ailsa (June 27, 2019). 'Zombies and Owls: How Atomic Cartoons Recruits Canada's Best Talent'. Royal Bank of Canada. Retrieved July 5, 2019.
- ^ abMiller, Bob (2000-09-01). 'The Power Behind Atomic Cartoons'. Animation World Network. Retrieved 2015-03-13.
- ^Goodman, Martin (2002-03-18). 'Atomic Betty: Defending the Universe and Trying to Find a Home on TV'. Animation World Network. Retrieved 2015-03-13.
- ^ abCummins, Juliana (July 8, 2015). 'Thunderbird acquires Atomic Cartoons'. Kidscreen. Brunico Communications. Retrieved April 8, 2019.
- ^Edwards, Ian (April 5, 1999). 'New Vancouver studio Atomic Cartoons opens'. Playback. Brunico Communications.
- ^Getzler, Wendy (October 19, 2011). 'Atomic Cartoons names head of production'. Kidscreen. Brunico Communications. Retrieved April 8, 2019.
- ^Pinto, Jordan (June 16, 2016). 'Twiner-McCarron named president of Atomic Cartoons'. Playback. Brunico Communications. Retrieved April 8, 2019.
- ^'Animation studio drawn back to Hintonburg'. CBC News. Canadian Broadcasting Corporation. December 16, 2018. Retrieved April 8, 2019.
- ^Malyk, Lauren (June 20, 2019). 'Atomic Cartoons moves into phase two of Ottawa expansion'. Playback. Brunico Communications. Retrieved July 5, 2019.
- ^Milligan, Mercedes (February 5, 2020). 'Thunderbird's Atomic Cartoons Opens LA Studio'. Animation Magazine. Retrieved February 6, 2020.
- ^https://atomiccartoons.com/the-walking-dead-creator-robert-kirkman-quietly-launched-a-tv-series-in-canada/
- ^Edwards, Ian (1999-11-15). 'Animal Planet calls on 'Wild''. Playback. Retrieved 2015-03-13.
- ^Godfrey, Leigh (October 25, 2001). 'Timberwolf On The Web'. Animation World Network. Retrieved February 6, 2020.
- ^Brodsky, Katherine (2012-09-05). 'World's love of toons makes these nerds cool'. Variety. Retrieved 2015-03-13.
- ^DeMott, Rick (2007-09-11). 'Captain Flamingo Lands On Jetix Programming Block'. Animation World Network. Retrieved 2015-03-13.
- ^Mallory, Michael (2012-06-14). 'The Tooning Up of 'Car Talk''. Animation Magazine. Retrieved 2015-03-13.
- ^https://atomiccartoons.com/atomic-cartoons-goes-boom/
- ^Wolfe, Jennifer (2014-10-15). 'TELETOON Commissions Third Season of Breakthrough's 'Rocket Monkeys''. Animation World Network. Retrieved 2015-03-13.
- ^https://www.awn.com/news/atomic-cartoons-revs-creative-investment
- ^Foster, Elizabeth (November 22, 2017). 'Spin Master's new dino-might'. Kidscreen. Brunico Communications. Retrieved November 22, 2017.
- ^Zahed, Ramin (August 13, 2020). 'Disney+ Gets Festive with 'LEGO Star Wars Holiday Special''. Animation Magazine. Retrieved August 27, 2020.
- ^Milligan, Mercedes (February 4, 2019). 'Tokidoki's Mermicorno Getting Animated with Atomic Cartoons'. Animation Magazine. Retrieved February 6, 2020.
- ^Tuchow, Ryan (February 25, 2020). 'How Jim Henson & Atomic are differentiating Nate Create'. Kidscreen. Brunico Communications. Retrieved February 25, 2020.
- ^Milligan, Mercedes (September 19, 2018). 'Atomic Cartoons Options Savannah Guthrie's 'Princesses Wear Pants' for Series'. Animation Magazine. Retrieved February 6, 2020.
- ^https://atomiccartoons.com/marblemedia-and-atomic-cartoons-enter-development-with-corus-entertainments-teletoon-on-animated-series/
- ^https://www.animationmagazine.net/tv/atomic-developing-mariachi-zombie/
External links[edit]
- Atomic Cartoons at IMDb
Click a column header, such as Name, to sort the table by that item.
SEENotes at the bottom of the Table.
| No. | Atomic weight | Name | Sym. | M.P. (°C) | B.P. (°C) | Density* (g/cm3) | Earth crust (%)* | Discovery (Year) | Group* | Electron configuration | Ionization energy (eV) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.008 | Hydrogen | H | -259 | -253 | 0.09 | 0.14 | 1776 | 1 | 1s1 | 13.60 | |
| 2 | 4.003 | Helium | He | -272 | -269 | 0.18 | 1895 | 18 | 1s2 | 24.59 | ||
| 3 | 6.941 | Lithium | Li | 180 | 1,347 | 0.53 | 1817 | 1 | [He] 2s1 | 5.39 | ||
| 4 | 9.012 | Beryllium | Be | 1,278 | 2,970 | 1.85 | 1797 | 2 | [He] 2s2 | 9.32 | ||
| 5 | 10.811 | Boron | B | 2,300 | 2,550 | 2.34 | 1808 | 13 | [He] 2s2 2p1 | 8.30 | ||
| 6 | 12.011 | Carbon | C | 3,500 | 4,827 | 2.26 | 0.09 | ancient | 14 | [He] 2s2 2p2 | 11.26 | |
| 7 | 14.007 | Nitrogen | N | -210 | -196 | 1.25 | 1772 | 15 | [He] 2s2 2p3 | 14.53 | ||
| 8 | 15.999 | Oxygen | O | -218 | -183 | 1.43 | 46.71 | 1774 | 16 | [He] 2s2 2p4 | 13.62 | |
| 9 | 18.998 | Fluorine | F | -220 | -188 | 1.70 | 0.03 | 1886 | 17 | [He] 2s2 2p5 | 17.42 | |
| 10 | 20.180 | Neon | Ne | -249 | -246 | 0.90 | 1898 | 18 | [He] 2s2 2p6 | 21.56 | ||
| 11 | 22.990 | Sodium | Na | 98 | 883 | 0.97 | 2.75 | 1807 | 1 | [Ne] 3s1 | 5.14 | |
| 12 | 24.305 | Magnesium | Mg | 639 | 1,090 | 1.74 | 2.08 | 1755 | 2 | [Ne] 3s2 | 7.65 | |
| 13 | 26.982 | Aluminum | Al | 660 | 2,467 | 2.70 | 8.07 | 1825 | 13 | [Ne] 3s2 3p1 | 5.99 | |
| 14 | 28.086 | Silicon | Si | 1,410 | 2,355 | 2.33 | 27.69 | 1824 | 14 | [Ne] 3s2 3p2 | 8.15 | |
| 15 | 30.974 | Phosphorus | P | 44 | 280 | 1.82 | 0.13 | 1669 | 15 | [Ne] 3s2 3p3 | 10.49 | |
| 16 | 32.065 | Sulfur | S | 113 | 445 | 2.07 | 0.05 | ancient | 16 | [Ne] 3s2 3p4 | 10.36 | |
| 17 | 35.453 | Chlorine | Cl | -101 | -35 | 3.21 | 0.05 | 1774 | 17 | [Ne] 3s2 3p5 | 12.97 | |
| 18 | 39.948 | Argon | Ar | -189 | -186 | 1.78 | 1894 | 18 | [Ne] 3s2 3p6 | 15.76 | ||
| 19 | 39.098 | Potassium | K | 64 | 774 | 0.86 | 2.58 | 1807 | 1 | [Ar] 4s1 | 4.34 | |
| 20 | 40.078 | Calcium | Ca | 839 | 1,484 | 1.55 | 3.65 | 1808 | 2 | [Ar] 4s2 | 6.11 | |
| 21 | 44.956 | Scandium | Sc | 1,539 | 2,832 | 2.99 | 1879 | 3 | [Ar] 3d1 4s2 | 6.56 | ||
| 22 | 47.867 | Titanium | Ti | 1,660 | 3,287 | 4.54 | 0.62 | 1791 | 4 | [Ar] 3d2 4s2 | 6.83 | |
| 23 | 50.942 | Vanadium | V | 1,890 | 3,380 | 6.11 | 1830 | 5 | [Ar] 3d3 4s2 | 6.75 | ||
| 24 | 51.996 | Chromium | Cr | 1,857 | 2,672 | 7.19 | 0.04 | 1797 | 6 | [Ar] 3d5 4s1 | 6.77 | |
| 25 | 54.938 | Manganese | Mn | 1,245 | 1,962 | 7.43 | 0.09 | 1774 | 7 | [Ar] 3d5 4s2 | 7.43 | |
| 26 | 55.845 | Iron | Fe | 1,535 | 2,750 | 7.87 | 5.05 | ancient | 8 | [Ar] 3d6 4s2 | 7.90 | |
| 27 | 58.933 | Cobalt | Co | 1,495 | 2,870 | 8.90 | 1735 | 9 | [Ar] 3d7 4s2 | 7.88 | ||
| 28 | 58.693 | Nickel | Ni | 1,453 | 2,732 | 8.90 | 0.02 | 1751 | 10 | [Ar] 3d8 4s2 | 7.64 | |
| 29 | 63.546 | Copper | Cu | 1,083 | 2,567 | 8.96 | ancient | 11 | [Ar] 3d10 4s1 | 7.73 | ||
| 30 | 65.390 | Zinc | Zn | 420 | 907 | 7.13 | ancient | 12 | [Ar] 3d10 4s2 | 9.39 | ||
| 31 | 69.723 | Gallium | Ga | 30 | 2,403 | 5.91 | 1875 | 13 | [Ar] 3d10 4s2 4p1 | 6.00 | ||
| 32 | 72.640 | Germanium | Ge | 937 | 2,830 | 5.32 | 1886 | 14 | [Ar] 3d10 4s2 4p2 | 7.90 | ||
| 33 | 74.922 | Arsenic | As | 81 | 613 | 5.72 | ancient | 15 | [Ar] 3d10 4s2 4p3 | 9.79 | ||
| 34 | 78.960 | Selenium | Se | 217 | 685 | 4.79 | 1817 | 16 | [Ar] 3d10 4s2 4p4 | 9.75 | ||
| 35 | 79.904 | Bromine | Br | -7 | 59 | 3.12 | 1826 | 17 | [Ar] 3d10 4s2 4p5 | 11.81 | ||
| 36 | 83.800 | Krypton | Kr | -157 | -153 | 3.75 | 1898 | 18 | [Ar] 3d10 4s2 4p6 | 14.00 | ||
| 37 | 85.468 | Rubidium | Rb | 39 | 688 | 1.63 | 1861 | 1 | [Kr] 5s1 | 4.18 | ||
| 38 | 87.620 | Strontium | Sr | 769 | 1,384 | 2.54 | 1790 | 2 | [Kr] 5s2 | 5.69 | ||
| 39 | 88.906 | Yttrium | Y | 1,523 | 3,337 | 4.47 | 1794 | 3 | [Kr] 4d1 5s2 | 6.22 | ||
| 40 | 91.224 | Zirconium | Zr | 1,852 | 4,377 | 6.51 | 0.03 | 1789 | 4 | [Kr] 4d2 5s2 | 6.63 | |
| 41 | 92.906 | Niobium | Nb | 2,468 | 4,927 | 8.57 | 1801 | 5 | [Kr] 4d4 5s1 | 6.76 | ||
| 42 | 95.940 | Molybdenum | Mo | 2,617 | 4,612 | 10.22 | 1781 | 6 | [Kr] 4d5 5s1 | 7.09 | ||
| 43 | * | 98.000 | Technetium | Tc | 2,200 | 4,877 | 11.50 | 1937 | 7 | [Kr] 4d5 5s2 | 7.28 | |
| 44 | 101.070 | Ruthenium | Ru | 2,250 | 3,900 | 12.37 | 1844 | 8 | [Kr] 4d7 5s1 | 7.36 | ||
| 45 | 102.906 | Rhodium | Rh | 1,966 | 3,727 | 12.41 | 1803 | 9 | [Kr] 4d8 5s1 | 7.46 | ||
| 46 | 106.420 | Palladium | Pd | 1,552 | 2,927 | 12.02 | 1803 | 10 | [Kr] 4d10 | 8.34 | ||
| 47 | 107.868 | Silver | Ag | 962 | 2,212 | 10.50 | ancient | 11 | [Kr] 4d10 5s1 | 7.58 | ||
| 48 | 112.411 | Cadmium | Cd | 321 | 765 | 8.65 | 1817 | 12 | [Kr] 4d10 5s2 | 8.99 | ||
| 49 | 114.818 | Indium | In | 157 | 2,000 | 7.31 | 1863 | 13 | [Kr] 4d10 5s2 5p1 | 5.79 | ||
| 50 | 118.710 | Tin | Sn | 232 | 2,270 | 7.31 | ancient | 14 | [Kr] 4d10 5s2 5p2 | 7.34 | ||
| 51 | 121.760 | Antimony | Sb | 630 | 1,750 | 6.68 | ancient | 15 | [Kr] 4d10 5s2 5p3 | 8.61 | ||
| 52 | 127.600 | Tellurium | Te | 449 | 990 | 6.24 | 1783 | 16 | [Kr] 4d10 5s2 5p4 | 9.01 | ||
| 53 | 126.905 | Iodine | I | 114 | 184 | 4.93 | 1811 | 17 | [Kr] 4d10 5s2 5p5 | 10.45 | ||
| 54 | 131.293 | Xenon | Xe | -112 | -108 | 5.90 | 1898 | 18 | [Kr] 4d10 5s2 5p6 | 12.13 | ||
| 55 | 132.906 | Cesium | Cs | 29 | 678 | 1.87 | 1860 | 1 | [Xe] 6s1 | 3.89 | ||
| 56 | 137.327 | Barium | Ba | 725 | 1,140 | 3.59 | 0.05 | 1808 | 2 | [Xe] 6s2 | 5.21 | |
| 57 | 138.906 | Lanthanum | La | 920 | 3,469 | 6.15 | 1839 | 3 | [Xe] 5d1 6s2 | 5.58 | ||
| 58 | 140.116 | Cerium | Ce | 795 | 3,257 | 6.77 | 1803 | 101 | [Xe] 4f1 5d1 6s2 | 5.54 | ||
| 59 | 140.908 | Praseodymium | Pr | 935 | 3,127 | 6.77 | 1885 | 101 | [Xe] 4f3 6s2 | 5.47 | ||
| 60 | 144.240 | Neodymium | Nd | 1,010 | 3,127 | 7.01 | 1885 | 101 | [Xe] 4f4 6s2 | 5.53 | ||
| 61 | * | 145.000 | Promethium | Pm | 1,100 | 3,000 | 7.30 | 1945 | 101 | [Xe] 4f5 6s2 | 5.58 | |
| 62 | 150.360 | Samarium | Sm | 1,072 | 1,900 | 7.52 | 1879 | 101 | [Xe] 4f6 6s2 | 5.64 | ||
| 63 | 151.964 | Europium | Eu | 822 | 1,597 | 5.24 | 1901 | 101 | [Xe] 4f7 6s2 | 5.67 | ||
| 64 | 157.250 | Gadolinium | Gd | 1,311 | 3,233 | 7.90 | 1880 | 101 | [Xe] 4f7 5d1 6s2 | 6.15 | ||
| 65 | 158.925 | Terbium | Tb | 1,360 | 3,041 | 8.23 | 1843 | 101 | [Xe] 4f9 6s2 | 5.86 | ||
| 66 | 162.500 | Dysprosium | Dy | 1,412 | 2,562 | 8.55 | 1886 | 101 | [Xe] 4f10 6s2 | 5.94 | ||
| 67 | 164.930 | Holmium | Ho | 1,470 | 2,720 | 8.80 | 1867 | 101 | [Xe] 4f11 6s2 | 6.02 | ||
| 68 | 167.259 | Erbium | Er | 1,522 | 2,510 | 9.07 | 1842 | 101 | [Xe] 4f12 6s2 | 6.11 | ||
| 69 | 168.934 | Thulium | Tm | 1,545 | 1,727 | 9.32 | 1879 | 101 | [Xe] 4f13 6s2 | 6.18 | ||
| 70 | 173.040 | Ytterbium | Yb | 824 | 1,466 | 6.90 | 1878 | 101 | [Xe] 4f14 6s2 | 6.25 | ||
| 71 | 174.967 | Lutetium | Lu | 1,656 | 3,315 | 9.84 | 1907 | 101 | [Xe] 4f14 5d1 6s2 | 5.43 | ||
| 72 | 178.490 | Hafnium | Hf | 2,150 | 5,400 | 13.31 | 1923 | 4 | [Xe] 4f14 5d2 6s2 | 6.83 | ||
| 73 | 180.948 | Tantalum | Ta | 2,996 | 5,425 | 16.65 | 1802 | 5 | [Xe] 4f14 5d3 6s2 | 7.55 | ||
| 74 | 183.840 | Tungsten | W | 3,410 | 5,660 | 19.35 | 1783 | 6 | [Xe] 4f14 5d4 6s2 | 7.86 | ||
| 75 | 186.207 | Rhenium | Re | 3,180 | 5,627 | 21.04 | 1925 | 7 | [Xe] 4f14 5d5 6s2 | 7.83 | ||
| 76 | 190.230 | Osmium | Os | 3,045 | 5,027 | 22.60 | 1803 | 8 | [Xe] 4f14 5d6 6s2 | 8.44 | ||
| 77 | 192.217 | Iridium | Ir | 2,410 | 4,527 | 22.40 | 1803 | 9 | [Xe] 4f14 5d7 6s2 | 8.97 | ||
| 78 | 195.078 | Platinum | Pt | 1,772 | 3,827 | 21.45 | 1735 | 10 | [Xe] 4f14 5d9 6s1 | 8.96 | ||
| 79 | 196.967 | Gold | Au | 1,064 | 2,807 | 19.32 | ancient | 11 | [Xe] 4f14 5d10 6s1 | 9.23 | ||
| 80 | 200.590 | Mercury | Hg | -39 | 357 | 13.55 | ancient | 12 | [Xe] 4f14 5d10 6s2 | 10.44 | ||
| 81 | 204.383 | Thallium | Tl | 303 | 1,457 | 11.85 | 1861 | 13 | [Xe] 4f14 5d10 6s2 6p1 | 6.11 | ||
| 82 | 207.200 | Lead | Pb | 327 | 1,740 | 11.35 | ancient | 14 | [Xe] 4f14 5d10 6s2 6p2 | 7.42 | ||
| 83 | 208.980 | Bismuth | Bi | 271 | 1,560 | 9.75 | ancient | 15 | [Xe] 4f14 5d10 6s2 6p3 | 7.29 | ||
| 84 | * | 209.000 | Polonium | Po | 254 | 962 | 9.30 | 1898 | 16 | [Xe] 4f14 5d10 6s2 6p4 | 8.42 | |
| 85 | * | 210.000 | Astatine | At | 302 | 337 | 0.00 | 1940 | 17 | [Xe] 4f14 5d10 6s2 6p5 | 9.30 | |
| 86 | * | 222.000 | Radon | Rn | -71 | -62 | 9.73 | 1900 | 18 | [Xe] 4f14 5d10 6s2 6p6 | 10.75 | |
| 87 | * | 223.000 | Francium | Fr | 27 | 677 | 0.00 | 1939 | 1 | [Rn] 7s1 | 4.07 | |
| 88 | * | 226.000 | Radium | Ra | 700 | 1,737 | 5.50 | 1898 | 2 | [Rn] 7s2 | 5.28 | |
| 89 | * | 227.000 | Actinium | Ac | 1,050 | 3,200 | 10.07 | 1899 | 3 | [Rn] 6d1 7s2 | 5.17 | |
| 90 | 232.038 | Thorium | Th | 1,750 | 4,790 | 11.72 | 1829 | 102 | [Rn] 6d2 7s2 | 6.31 | ||
| 91 | 231.036 | Protactinium | Pa | 1,568 | 0 | 15.40 | 1913 | 102 | [Rn] 5f2 6d1 7s2 | 5.89 | ||
| 92 | 238.029 | Uranium | U | 1,132 | 3,818 | 18.95 | 1789 | 102 | [Rn] 5f3 6d1 7s2 | 6.19 | ||
| 93 | * | 237.000 | Neptunium | Np | 640 | 3,902 | 20.20 | 1940 | 102 | [Rn] 5f4 6d1 7s2 | 6.27 | |
| 94 | * | 244.000 | Plutonium | Pu | 640 | 3,235 | 19.84 | 1940 | 102 | [Rn] 5f6 7s2 | 6.03 | |
| 95 | * | 243.000 | Americium | Am | 994 | 2,607 | 13.67 | 1944 | 102 | [Rn] 5f7 7s2 | 5.97 | |
| 96 | * | 247.000 | Curium | Cm | 1,340 | 0 | 13.50 | 1944 | 102 | 5.99 | ||
| 97 | * | 247.000 | Berkelium | Bk | 986 | 0 | 14.78 | 1949 | 102 | 6.20 | ||
| 98 | * | 251.000 | Californium | Cf | 900 | 0 | 15.10 | 1950 | 102 | 6.28 | ||
| 99 | * | 252.000 | Einsteinium | Es | 860 | 0 | 0.00 | 1952 | 102 | 6.42 | ||
| 100 | * | 257.000 | Fermium | Fm | 1,527 | 0 | 0.00 | 1952 | 102 | 6.50 | ||
| 101 | * | 258.000 | Mendelevium | Md | 0 | 0 | 0.00 | 1955 | 102 | 6.58 | ||
| 102 | * | 259.000 | Nobelium | No | 827 | 0 | 0.00 | 1958 | 102 | 6.65 | ||
| 103 | * | 262.000 | Lawrencium | Lr | 1,627 | 0 | 0.00 | 1961 | 102 | 4.90 | ||
| 104 | * | 261.000 | Rutherfordium | Rf | 0 | 0 | 0.00 | 1964 | 4 | 0.00 | ||
| 105 | * | 262.000 | Dubnium | Db | 0 | 0 | 0.00 | 1967 | 5 | 0.00 | ||
| 106 | * | 266.000 | Seaborgium | Sg | 0 | 0 | 0.00 | 1974 | 6 | 0.00 | ||
| 107 | * | 264.000 | Bohrium | Bh | 0 | 0 | 0.00 | 1981 | 7 | 0.00 | ||
| 108 | * | 277.000 | Hassium | Hs | 0 | 0 | 0.00 | 1984 | 8 | 0.00 | ||
| 109 | * | 268.000 | Meitnerium | Mt | 0 | 0 | 0.00 | 1982 | 9 | 0.00 | ||
| No. | Atomic weight | Name | Sym. | M.P. (°C) | B.P. (°C) | Density* (g/cm3) | Earth crust (%)* | Discovery (Year) | Group* | Electron configuration | Ionization energy (eV) |
Notes:
• Density of elements with boiling points below 0°C is given in g/l. In a sorted list, these elements are shown before other elements that have boiling points >0°C.
• Earth crust composition average values are from a report by F. W. Clarke and H. S. Washington, 1924. Elemental composition of crustal rocks differ between different localities (see article).
• Group: There are only 18 groups in the periodic table that constitute the columns of the table. Lanthanoids and Actinoids are numbered as 101 and 102 to separate them in sorting by group.
• The elements marked with an asterisk (in the 2nd column) have no stable nuclides. For these elements the weight value shown represents the mass number of the longest-lived isotope of the element.

Abbreviations and Definitions:
No. - Atomic Number; M.P. - melting point; B.P. - boiling point
Atomic number: The number of protons in an atom. Each element is uniquely defined by its atomic number.
Atomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Atomic mass is measured in Atomic Mass Units (amu) which are scaled relative to carbon, 12C, that is taken as a standard element with an atomic mass of 12. This isotope of carbon has 6 protons and 6 neutrons. Thus, each proton and neutron has a mass of about 1 amu.
Atomic Number 13
Isotope: Atoms of the same element with the same atomic number, but different number of neutrons. Isotope of an element is defined by the sum of the number of protons and neutrons in its nucleus. Elements have more than one isotope with varying numbers of neutrons. For example, there are two common isotopes of carbon, 12C and 13C which have 6 and 7 neutrons respectively. The abundances of different isotopes of elements vary in nature depending on the source of materials. For relative abundances of isotopes in nature see reference on Atomic Weights and Isotopic Compositions.
Atomic weight: Atomic weight values represent weighted average of the masses of all naturally occurring isotopes of an element. The values shown here are based on the IUPAC Commission determinations (Pure Appl. Chem. 73:667-683, 2001). The elements marked with an asterisk have no stable nuclides. For these elements the weight value shown represents the mass number of the longest-lived isotope of the element.
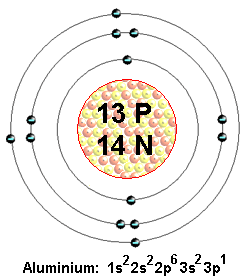
Electron configuration: See next page for explanation of electron configuration of atoms.
Ionization energy (IE): The energy required to remove the outermost electron from an atom or a positive ion in its ground level. The table lists only the first IE in eV units. To convert to kJ/mol multiply by 96.4869. Reference: NIST Reference Table on Ground states and ionization energies for the neutral atoms. IE decreases going down a column of the periodic table, and increases from left to right in a row. Thus, alkali metals have the lowest IE in a period and Rare gases have the highest.
Other resources related to the Periodic Table
- Chemical Evolution of the Universe
